Breadcrumb
- Home
- Serotonin Research
Serotonin Neurons & pH Homeostasis
Medullary serotonergic neurons are sensors for CO2 and pH.
Blood pH is tightly controlled, because even small changes in pH can be fatal. One of the major mechanisms for this pH
regulation is via regulation of carbon dioxide levels, since CO2 and pH are in equilibrium through the chemical reaction H2O + CO2 ⇔ H+ + HCO3-.
To regulate CO2, there are neurons in the brain called central respiratory chemoreceptors that monitor pH/CO2 and alter the rate and depth of lung ventilation to
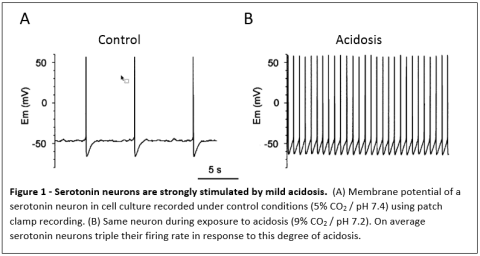
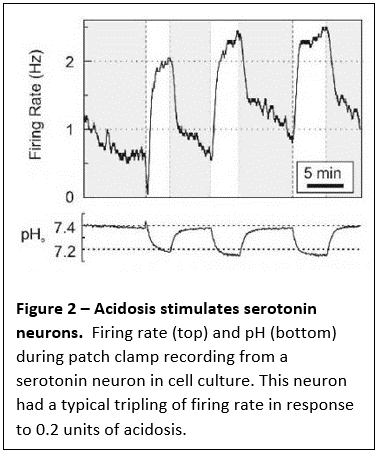
maintain them constant. We have shown that neurons in the medulla that produce serotonin (serotonergic neurons) have properties expected of central respiratory chemoreceptors. For example, they are strongly stimulated by an increase in CO2 via the resulting decrease in pH (Figure 1 and 2).
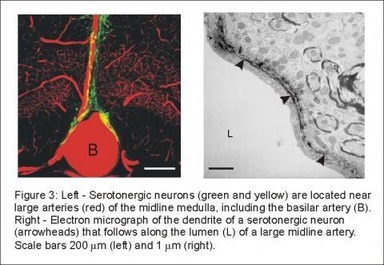
Serotonergic neurons in the medulla are also closely associated with large branches of the basilar artery (Figure 3). This is an ideal location for central respiratory chemoreceptors, because the CO2 of blood in these large arteries would not yet have been altered by tissue metabolism, so that the level of CO2 would more closely reflect the effectiveness of lung ventilation than CO2 of blood in capillaries or veins.
Serotonergic neurons as central respiratory chemoreceptors
There is now a variety of data from our laboratory and others using in vivo experiments that support a role of medullary serotonergic neurons as central respiratory chemoreceptors.
Our current work is aimed at studying the properties of serotonergic neurons to understand how they carry out their function as central chemoreceptors. To define these properties and their mechanisms we are using a combination of patch clamp recordings from brain slices and tissue culture, intracellular pH imaging, immunohistochemistry, and molecular biology. Our major goals are to define the mechanisms of pH chemosensitivity and how serotonergic neurons modulate downstream neurons in response to acidosis.
Midbrain serotonergic neurons are also sensors for CO2 and pH

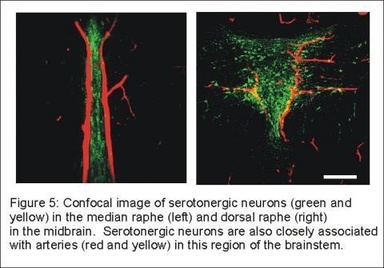
Our initial work focused on the role of medullary serotonergic neurons in respiratory control. However, serotonergic neurons in the midbrain have many properties in common with those in the medulla. Therefore, we examined the effect of CO2 / pH on midbrain serotonergic neurons and found that they are also highly sensitive to changes in CO2 and pH (Figure 4). Midbrain serotonergic neurons are also closely associated with large branches of the basilar artery that penetrate the midline of the midbrain (Figure 5).
Thus, midbrain serotonergic neurons also appear to be central
chemoreceptors. However, they are not involved in control of breathing. Instead, midbrain serotonergic neurons project to the forebrain and are involved in arousal, limbic function and cerebrovascular control. We have proposed that these chemosensitive midbrain serotonergic neurons mediate the arousal, anxiety and changes in cerebral blood flow that are known to occur when blood CO2 rises. In vivo experiments have demonstrated that selective stimulation of midbrain serotonin neurons with focal acidosis causes arousal from sleep, and selective deletion of these neurons prevents arousal from sleep in response to hypercapnia.
Do serotonergic neurons throughout the brainstem share a role as CO2 / pH sensors?
Since the majority (70-90%) of serotonergic neurons in the medulla are stimulated by acidosis, some of these neurons must project to nonrespiratory nuclei. If this is the case, then what do these neurons do? How does this relate to the fact that the majority of serotonergic neurons in the midbrain are also chemosensitive? Many of the neural systems influenced by serotonergic neurons are also sensitive to changes in blood CO2, so it is reasonable to suppose that 5-HT neurons mediate nonrespiratory effects of increased CO2.
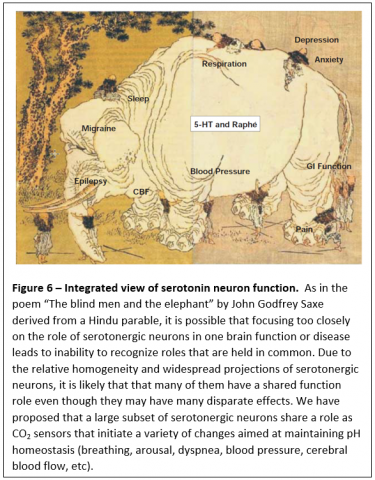
Our hypothesis is that chemosensitive serotonergic neurons induce a variety of downstream effects aimed at restoring pH homeostasis in response to a CO2 challenge, including increased ventilation, changes in autonomic output, arousal, anxiety and altered cerebrovascular flow.
Serotonin neurons are also important for brown fat metabolism and motor activity that controls body temperature (e.g. shivering). It is not clear whether that is unrelated to their role in pH control, or whether these neurons could be involved more broadly in integrating body metabolism which includes oxygen handling, body temperature, pH, etc.
Serotonin and human disease
The overall goal of our lab is to determine the mechanisms by which serotonergic neurons sense changes in CO2, and how their downstream effects contribute to control of pH. Defining the mechanisms of central respiratory chemoreception may lead to specific treatments for diseases in which respiratory chemoreception is abnormal and provide a better understanding of how CO2 and pH affect CNS function.
For more detailed information about the work described here, please see the following papers:
Wu, Y., K. Proch, F.A. Teran, R. Lechtenberg, H. Kothari & G.B. Richerson. Input from serotonin neurons contributes to chemosensitivity of Phox2b expressing retrotrapezoid neurons. J Physiol 597(10):2741-2766, 2019. [PMID: 30866045]
Cerpa, V.J., Y. Wu, E.U. Bravo, F.A. Teran, R.S. Flynn & G.B. Richerson. Medullary 5-HT neurons: Switch from respiratory drive to chemoreception during postnatal development. Neurosci, 344:1-14, 2017. [PMID: 27619736]
McGlashon, J.B., A. Kozlowski, M. Gorecki, C. Thirnbeck, K. Markan, M.E. Kotas, M.J. Potthoff, G.B. Richerson & M.P. Gillum. Central serotonergic neurons activate and recruit thermogenic brown and beige fat and regulate glucose and lipid homeostasis. Cell Metabolism, 21(5):692-705, 2015. [PMID: 25955206] PMCID: PMC4565052.
Brust, R.D., A.E. Corcoran, G.B. Richerson, E.E.Nattie, S.M. Dymecki. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Reports, 9(6):2152-65, 2014. [PMID: 25497093] PMCID:PMC4351771.
Teran, F.A., C.A. Massey & G.B. Richerson. Serotonin neurons and central respiratory chemoreception: Where are we now? Prog Brain Res, 209:207-233, 2014. [PMID: 24746050] PMCID: PMC4486262.
Ray, R., A. Corcoran, R. Brust, J. C. Kim, G.B. Richerson, E.E. Nattie & S. M. Dymecki. Homeostatic imbalance upon acute in vivo serotonergic neuron inhibition. Science 333(6042): 637-642, 2011. [PMID: 21798952] PMCID: PMC3729433 (Science Editor’s Choice). (Faculty of 1000).
Buchanan, G.F. & G.B. Richerson. Central serotonin neurons are required for arousal to CO2. PNAS 107(37): 16354-9, 2010. [PMID: 20805497] PMCID: PMC2941296.
Hodges, M.R., M. Wehner, J. Aungst, J.C. Smith & G.B. Richerson. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci 29(33):10341-10349, 2009. [PMID: 19692608] PMCID: PMC2755228.
Ptak, K., T. Yamanishi, J. Aungst, L.S. Milescu, R. Zhang, G.B. Richerson & J.C. Smith. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29(12): 3720-37, 2009. PMCID: PMC2940110.
Hodges, M.R., G.J. Tattersall, M.B. Harris, S.D. McEvoy, D.N. Richerson, E.S. Deneris, R.L. Johnson, Z.F. Chen & G.B. Richerson. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28(10): 2495-2505, 2008. [PMID: 18322094]
Richerson, G.B. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nature Reviews Neuroscience 5:449-461, 2004.
Severson, C.A., W. Wang, V.A. Pieribone, C.I. Dohle & G.B. Richerson. Midbrain serotonergic neurons are central pH chemoreceptors. Nature Neurosci 6(11):1139-1140, 2003.
Bradley, S. Risso, V. A. Pieribone, W. Wang, C.A. Severson, R. A. Jacobs & G. B. Richerson. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nature Neurosci 5(5): 401-402, 2002.
Wang, W., S. Risso Bradley & G.B. Richerson. Quantification of the response of rat medullary raphe neurones to independent changes in pHo and PCO2. J Physiol (Lond) 540(3): 951-970, 2002.
Wang, W., A.V. Zaykin, J.K. Tiwari, S. Risso Bradley & G.B. Richerson. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol 85:2224-2235, 2001.
Richerson, G.B. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol 73(3):933-944, 1995.
Dekin, M.S., G.B. Richerson & P.A. Getting. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science 229:67-69, 1985.